Mining the CRBN target space redefines rules for molecular glue-induced neosubstrate recognition.
Petzold, G., Gainza, P., Annunziato, S., Lamberto, I., Trenh, P., McAllister, L.A., DeMarco, B., Schwander, L., Bunker, R.D., Zlotosch, M., SriRamaratnam, R., Gilberto, S., Langousis, G., Donckele, E.J., Quan, C., Strande, V., De Donatis, G.M., Alabi, S.B., Alers, J., Matysik, M., Staehly, C., Dubois, A., Osmont, A., Garskovas, M., Lyon, D., Wiedmer, L., Oleinikovas, V., Lieberherr, R., Rubin, N.T., Lam, D.T., Lucas, X., Liardo, E., Widlund, N.I., Ritzen, A., Caceres, R.M., Vigil, D., Tsai, J., Wallace, O., Peluso, M., Sadok, A., Tiedt, R., Paterson, A.M., Zarayskiy, V., Fasching, B., Bonenfant, D., Warmuth, M., Castle, J.C., Townson, S.A.(2025) Science 389: eadt6736-eadt6736
- PubMed: 40608931
- DOI: https://doi.org/10.1126/science.adt6736
- Primary Citation of Related Structures:
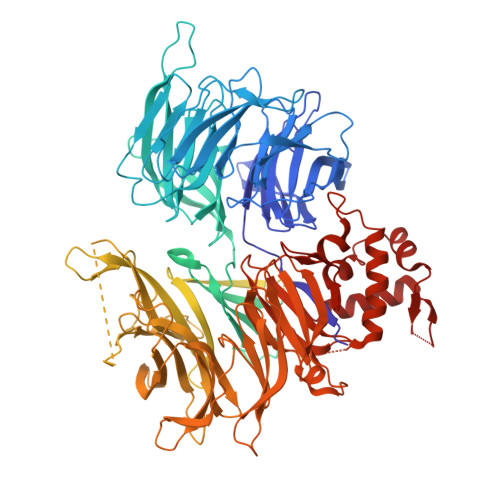
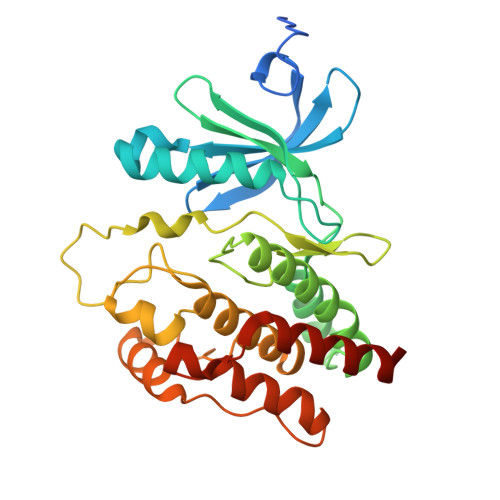
9NFQ, 9NFR, 9NGT - PubMed Abstract:
The CRL4 CRBN E3 ubiquitin ligase is the target of molecular glue degrader compounds that reprogram ligase specificity to induce the degradation of clinically relevant neosubstrate proteins. Known cereblon (CRBN) neosubstrates share a generalizable β-hairpin G-loop recognition motif that allows for the systematic exploration of the CRBN target space. Computational mining approaches using structure- and surface-based matchmaking algorithms predict more than 1600 CRBN-compatible G-loop proteins across the human proteome, including the newly discovered helical G-loop motif, and identify the noncanonical neosubstrate binding mode of VAV1 that engages CRBN through a molecular surface mimicry mechanism. This work broadens the CRBN target space, redefines rules for neosubstrate recognition, and establishes a platform for the elimination of challenging drug targets by repurposing CRL4 CRBN through next-generation molecular glue degraders.
- Monte Rosa Therapeutics Inc, Boston, MA, USA.
Organizational Affiliation: