BceABS nucleotide-free state
McAndrew, M.B.L., Brotherton, D.H., Stansfeld, P.J., Crow, A.To be published.
Experimental Data Snapshot
Starting Model: in silico
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Bacitracin export permease protein BceB | 646 | Bacillus subtilis subsp. subtilis str. 168 | Mutation(s): 0 Gene Names: bceB, barD, ytsD, BSU30370 Membrane Entity: Yes | 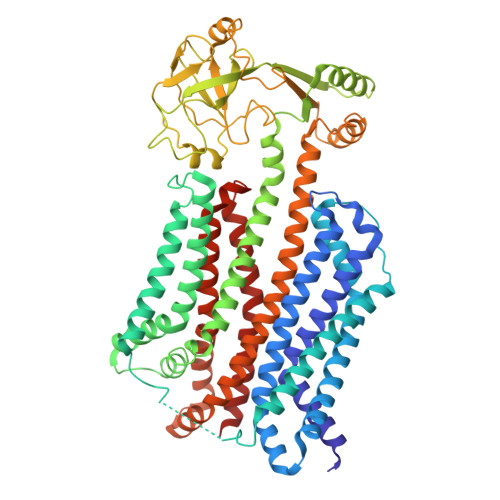 | |
UniProt | |||||
Find proteins for O34741 (Bacillus subtilis (strain 168)) Explore O34741 Go to UniProtKB: O34741 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O34741 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Bacitracin export ATP-binding protein BceA | 273 | Bacillus subtilis subsp. subtilis str. 168 | Mutation(s): 0 Gene Names: bceA, barC, ytsC, BSU30380 |  | |
UniProt | |||||
Find proteins for O34697 (Bacillus subtilis (strain 168)) Explore O34697 Go to UniProtKB: O34697 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O34697 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Sensor protein BceS | 334 | Bacillus subtilis subsp. subtilis str. 168 | Mutation(s): 0 Gene Names: bceS, barB, ytsB, BSU30390 EC: 2.7.13.3 Membrane Entity: Yes | 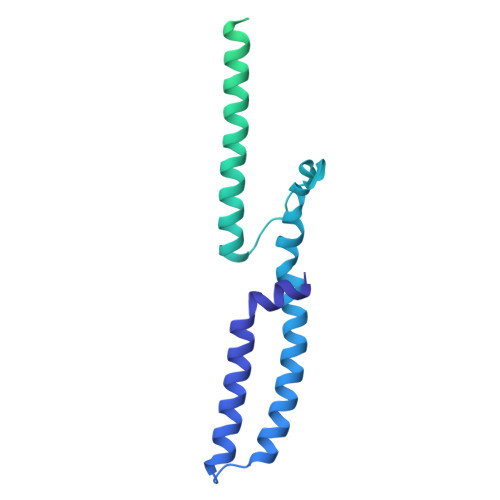 | |
UniProt | |||||
Find proteins for O35044 (Bacillus subtilis (strain 168)) Explore O35044 Go to UniProtKB: O35044 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O35044 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| CDL Query on CDL | G [auth E] | CARDIOLIPIN C81 H156 O17 P2 XVTUQDWPJJBEHJ-KZCWQMDCSA-L |  | ||
| 3PE Query on 3PE | F [auth D] | 1,2-Distearoyl-sn-glycerophosphoethanolamine C41 H82 N O8 P LVNGJLRDBYCPGB-LDLOPFEMSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| RECONSTRUCTION | RELION | 5-beta |
| MODEL REFINEMENT | PHENIX | 1.20.1 |
| MODEL REFINEMENT | ISOLDE |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Medical Research Council (MRC, United Kingdom) | United Kingdom | MR/N014294/1 |